🧬 What Exactly is an Atom?
Atoms are the smallest building blocks of matter. Everything around you — your phone, food, books, even you — is made of atoms!
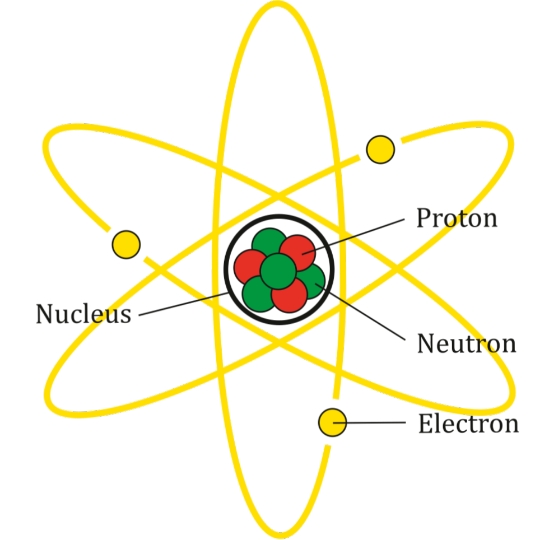
Think of atoms like tiny solar systems:
At the center is the nucleus (contains protons and neutrons)
Around it spin the electrons in orbits
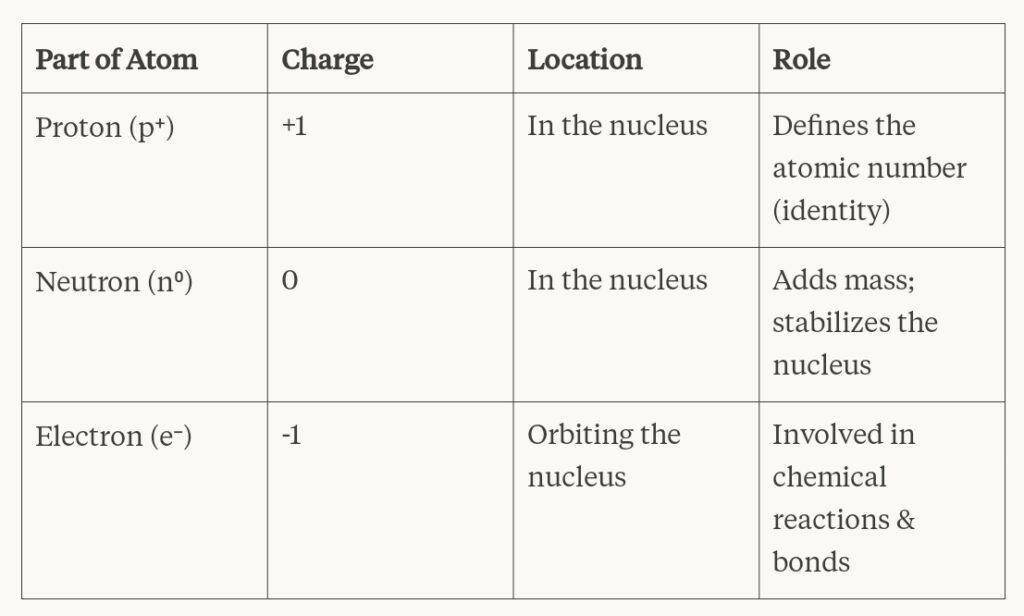
🔍 Structure of an Atom
🤯 Mind-Blowing Facts About Atoms
🌠 You’re made of stardust! Most atoms in your body were formed in stars billions of years ago.
🕳️ Mostly empty: Atoms are 99.9999999% empty space.
🧮 Tiny but mighty: A single grain of sand holds quintillions of atoms.
🔄 Atoms never die: In a chemical reaction, atoms aren’t destroyed — just rearranged.
🧪 How Atoms Combine: Molecules & Compounds
Atoms like to stay in groups. They bond together to form molecules and compounds.
Here are some examples:
💧 H₂O – Water → 2 hydrogen atoms + 1 oxygen atom
🍃 CO₂ – Carbon dioxide → 1 carbon + 2 oxygen atoms
🍬 C₆H₁₂O₆ – Glucose → sugar molecule made of carbon, hydrogen, and oxygen
👉 These combinations give every substance its unique properties.
🌱 Where Do You See Atoms in Real Life?
Atoms are literally everywhere. Here’s how they matter in your daily life:
💊 Medicines interact with atoms in your body
💡 Electricity flows due to moving electrons (part of atoms)
📱 Gadgets like phones are made with semiconductors at atomic levels
⚛️ Nuclear energy is produced by splitting atoms
🎯 Recap: Why Atoms Are Amazing
✔️ All matter is made of atoms
✔️ Atoms contain protons, neutrons, and electrons
✔️ Atoms combine to form molecules and life
✔️ Understanding atoms = understanding the universe!
🧠 Quick Quiz Time!
💡 This atom has 1 proton, no neutron, and 1 electron. What is it?
👇
👇
🎉 Answer: Hydrogen